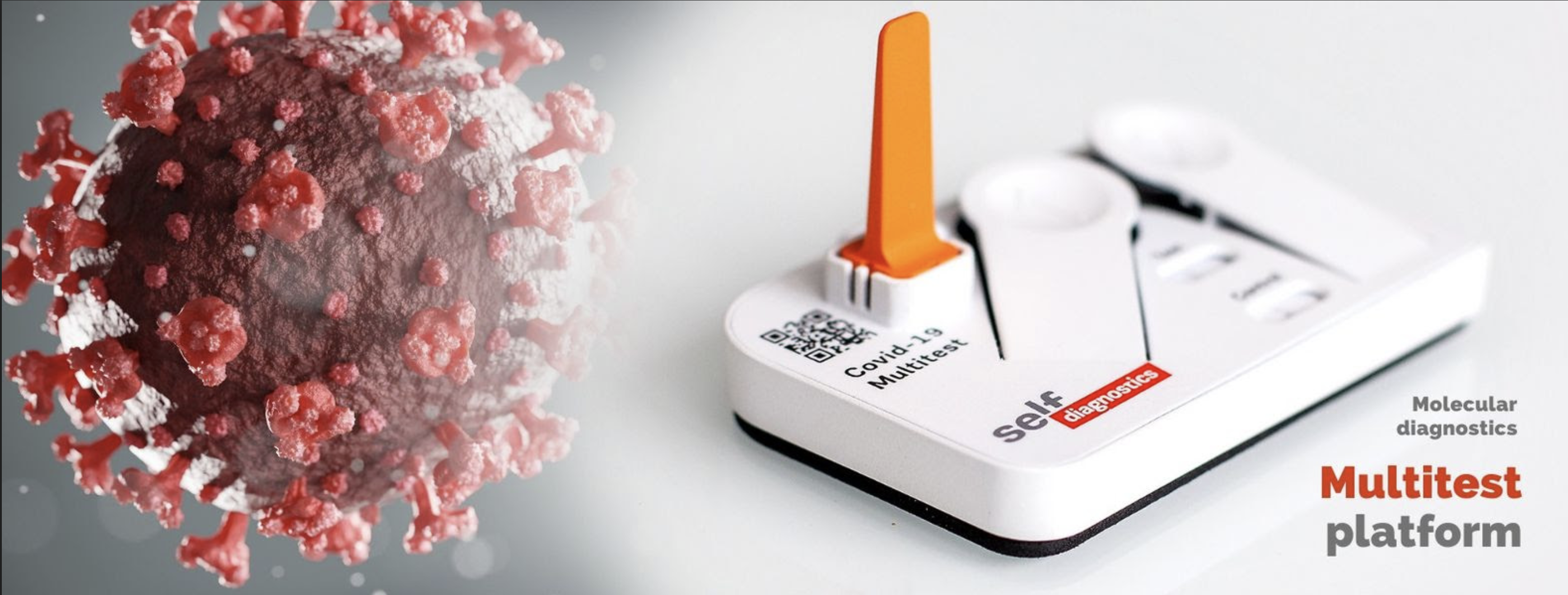
After years of research and development, Selfdiagnostics brings a portable and pocket-size test to the market to detect SARS-CoV-2 in 45 minutes. The CE marked Multitest COVID-19 is registered in German Medical Device Information and Database System (DMIDS) and in European Database on Medical Devices (EUDAMED). FDA and other certifications will follow.
The 2019 outbreak of novel coronavirus (SARS-CoV-2) was declared a Public Health Emergency of International Concern on January 30, 2020 by the World Health Organisation (WHO). In recognition of the acute necessity for proper diagnosis, Selfdiagnostics developed Multitest Covid-19 to detect the virus with high accuracy and speed without the need of expensive lab equipment and long waiting periods.