Scalable cutting edge rapid testing technology
From 10 years to
From 3 weeks to
From 50 percentage to
Our technology allows developing detection of new pathogens within 1 year instead of 10 years as it has been now.
Rapid tests with our technology allow testing within 1 week after infection instead of 3 weeks as it is now.
We have increased rapid testing accuracy to lab level.
NINAAT technology
Our NINAAT (Non-Instrumented Nucleic Acid Amplification) molecular diagnostics technology can be used as a solid and reliable foundation, which can easily be adapted for military, veterinary, food, agriculture, pharmaceuticals or cosmetic needs. Everywhere, where the detection of viral, bacterial and protozoan infections or any genetic marker are required, our platform gives you the most advanced technology to do it.
Main advantages of NINAAT platform
- Accuracy equal to laboratory
- Gives multiplex results for better diagnosis
- Testing procedure so easy it can also be used in self tests
- Quickly adaptable for any new target – within months
- Results immediately after infection, not after immune reaction of body
How does the NINAAT technology work – the details.
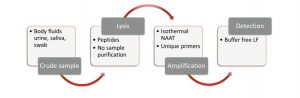
Selfdiagnostics possesses unique proprietary technology for nucleic acid detection embedded into NINAAT platform enabling the application by untrained lay persons. The technology is more robust, effective and shows better sensitivity compared to lateral flow based home tests.
Technology uses crude sample; no purification is required. As a result, the platform avoids complicated and time-consuming DNA extraction procedures. The amplification process is isothermal, it does not require neither temperature cycles nor instrumentation.
The technological invention of Selfdiagnostics is combination of a robust sample preparation, specific DNA detection and isothermal amplification with simple lateral flow visualisation that are all combined with self-regulating micro-heater and operated by passive microfluidics. Thus, no specific instruments are needed, all these components are integrated and optimised into a small sized, fully disposable device.
Our partners
During open innovation technology development Selfdiagnostics is or has been collaborating with many academic institutions in Europe – University of Tartu, Fraunhofer Institute for Cell Therapy and Immunology (IZI), Tallinn University of Technology, Dresden University of Technology, Institute for Bioprocessing and Analytical Measurement Techniques, and with many companies in Europe (in Estonia, Germany, United Kingdom) and in US and Asia.
Our patents
Selfdiagnostics NINAAT technology is protected by 7 patent families with priority dates from 2010 to 2017 in most strategic markets including Europe, USA, Japan, Australia and Canada. The Background IP covers all fundamental methods and their composition.
- “Method and rapid test device for the detection of target molecule”; submitted to authorities in the US and Europe with priority date (29 Jan 2010) and the purpose to protect both the method and a disposable device for POC products utilising amplification methods, on-board heating and pretreatment from undiluted urine.
- “Method and its compositions for detection of nucleic acid target from biological samples and body fluids”; submitted to authorities in the US, Canada, Australia, Japan and Europe with priority date (20 Oct 2012) with the purpose to protect developed primers, reactions, and all other conditions.
- “Method for the detection of a sexually transmitted infectious pathogen”; submitted to PCT authorities for international evaluation with priority date (10 Dec 2015) with the purpose to protect Chlamydia trachomatis assay and its related components.
- “Microfluidic test device”; submitted to authorities in Denmark with priority date (22 Feb 2017) with the purpose to protect microfluidic cell (CoreChip) of POC device applications.
- “Sample plug with sealing part and related method”; submitted to authorities in Denmark with priority date (22 Feb 2017) with the purpose to protect sample plug sealing and its related method.
- ”Sample plug with sample recess and related method”; submitted to authorities in Denmark with priority date (22 Feb 2017) with the purpose to protect sample measuring plug and its related method.
- ”LAMP component distribution in an microfluidic cell”; submitted to authorities in Denmark with priority date (04 Apr 2017) with the purpose to protect regent component distribution and positioning in microfluidic cell (CoreChip).
Publications
In addition to patents, Selfdiagnostics and its workers have published numerous scientific papers with partners.
- Jevtuševskaja J. Application of isothermal amplification methods for detection of Chlamydia trachomatis directly from biological samples. University of Tartu Press, 2017.
- Jevtuševskaja J, Krõlov K, Tulp I, Langel Ü. The effect of main urine inhibitors on the activity of different DNA polymerases in loop-mediated isothermal amplification. Expert Rev Mol Diagn. 2017 Apr;17(4):403-410.
- Pardy, Tamas; Rang, Toomas; Tulp, Indrek (2016). Finite Element Modelling For The Optimization Of Microheating In Disposable Molecular Diagnostics. 5: Heat Transfer 2016, Ancona, Italy, September 7-9 September, 2016. WIT Press, 13−22. (International Journal of Computational Methods and Experimental Measurements; 106).
- Pardy, Tamas; Rang, Toomas; Tulp, Indrek (2016). Modelling and experimental characterisation of thermoelectric heating for molecular diagnostics devices. IEEE: 15th Biennial Baltic Electronics Conference (BEC), 2016. IEEE Xplore: IEEE, 27−30.
- Jevtuševskaja J, Uusna J, Andresen L, Krõlov K, Laanpere M, Grellier T, Tulp I, Langel Ü. Combination with antimicrobial peptide lyses improves loop-mediated isothermal amplification based method for Chlamydia trachomatis detection directly in urine sample. BMC Infect Dis. 2016 Jul 13;16:329.
- Pardy, Tamas; Rang, Toomas; Tulp, Indrek (2015). Modelling and experimental characterization of self-regulating resistive heating elements for disposable medical diagnostics devices. In: C. A. Brebbia. Materials Characterization VII (263−271). Great Britain: WIT Press.
- Tamás, Pardy; Toomas, Rang; Indrek, Tulp (2015). Finite element modelling of the resistive heating of disposable molecular diagnostics devices. WIT Transactions on Modelling and Simulation, 59, 381−391.
- Krõlov K, Frolova J, Tudoran O, Suhorutsenko J, Lehto T, Sibul H, Mäger I, Laanpere M, Tulp I, Langel Ü. Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples. J Mol Diagn. 2014 Jan;16(1):127-35.
- Garcia-Sosa, Alfonso T.; Tulp, Indrek; Langel, Kent; Langel, Ülo (2014). Peptide-ligand binding modeling of siRNA with cell-penetrating peptides. BioMed Research International, 257040, 10.1155/2014/257040.
- Gergely Huszka, Master’s Degree thesis, 2013, (sup) Prof. Mart Min and Indrek Tulp, Development of a microfluidic platform for nucleic acid amplification tests, Tallinn University of Technology, Faculty of Information Technology, Thomas Johann Seebeck Department of Electronics.
- Viikov, K.; Jasnovidova, O.; Tamm, T.; Sedman, J. (2012). C-Terminal Extension of the Yeast Mitochondrial DNA Polymerase Determines the Balance between Synthesis and Degradation. PLoS ONE, 7(3), e33482.
- Lehto, T; Kurrikoff, K; Langel, Ü. (2012). Cell-penetrating peptides for the delivery of nucleic acids. Expert Opinion on Drug Delivery, 9(7), 823 – 836.
- Mann, A.; Thakur, G.; Shukla, V.; Singh, A. K.; Khanduri, R.; Naik, R.; Jiang, Y.; Kalra, N.; Dwarakanath, B. S.; Langel, Ü.; Ganguli, M. (2011). Differences in DNA condensation and release by lysine and arginine homopeptides govern their DNA delivery efficiencies. Molecular Pharmaceutics, 8(5), 1729 – 1741.
- Viikov, K.; Väljamäe, P.; Sedman, J. (2011). Yeast mitochondrial DNA polymerase is a highly processive single-subunit enzyme. Mitochondrion, 11(1), 119 – 126.
- Dobchev, D. A.; Mäger, I.; Tulp, I.; Karelson, G.; Tamm, T.; Tämm, T.; Jänes, J.; Langel, Ü.; Karelson, M. (2010). Prediction of Cell-Penetrating Peptides Using Artificial Neural Networks. Current Computer-Aided Drug Design, 6(2), 79 – 89.
- Kilk, K.; Mahlapuu, R.; Soomets, U.; Langel, Ü. (2009). Analysis of in vitro toxicity of five cell-penetrating peptides by metabolic profiling.Toxicology, 265(3), 87 – 95.
- Karelson, M.; Karelson, G.; Tamm, T.; Tulp, I.; Jänes, J.; Tämm, K.; Lomaka, A.; Savchenko, D.; Dobchev, D.A. (2009). QSAR study of pharmacological permeabilities. Arkivoc, 218 – 238.
- Fuxe, K.; Canals, M.; Torvinen, M.; Marcellino, D.; Terasmaa, A.; Genedani, S.; Leo, G.; Guidolin, G.; Diaz-Cabiale, Z.; Rivera, A.; Lundström, L.; Langel, Ü.; Narvaez, J.; Tanganelli, S.; Lluis, C.; Ferre, S.; Woods, A.; Franco, R.; Agnati, LF. (2007). Intramembrane receptor-receptor interactions: a novel principle in molecular medicine. Journal of Neural Transmission, 114(1), 49 – 75.
- Saal, K.; Tätte, T.; Tulp, I.; Kink, I.; Kurg, A.; Mäeorg, U.; Rinken, A.; Lõhmus, A. (2006). Sol-gel films for DNA microarray applications. Materials Letters, 60(15), 1833 – 1838.
- Baumann, C.; Boehden, GS.; Burkle, A.; Wiesmüller, L. (2006). Poly(ADP-RIBOSE) polymerase-1 (Parp-1) antagonizes topoisomerase I-dependent recombination stimulation by P53. Nucleic Acids Res., 9;34(3), 1036 – 49.
Posters:
- V. Tröger et al., “Development of a nucleic acid-based amplification test as point-of-care rapid test detecting sexually transmitted diseases for home-care,” presented at the Point-of-Care Diagnostics & Global Health World Congress Agenda, San Diego, San Diego, 2015. (URL: https://www.eposters.net/poster/development-of-a-nucleic-acid-based-amplification-test-as-point-of-care-rapid-test-detecting)
- Pardy Tamas, Natalja Sleptsuk, Mart Min, Jaan Ojarand, Triin Lakspere, and Kaia Palm, “Rapid electrochemical impedance spectroscopy for protein detection in Lab-on-a-Chip devices,” presented at the Lab-on-a-Chip & Microfluidics 2016, Madrid, Spain, 2016. (URL: https://www.eposters.net/poster/rapid-electrochemical-impedance-spectroscopy-for-protein-detection-in-lab-on-a-chip-devices)
- Pardy Tamas, Toomas Rang, and Indrek Tulp, “Finite element modelling for the optimization of temperature control in Lab-on-a-Chip devices,” presented at the Lab-on-a-Chip & Microfluidics 2016, Madrid, Spain, 2016. (URL: https://www.eposters.net/poster/finite-element-modelling-for-the-optimization-of-temperature-control-in-lab-on-a-chip-devices-1)



