Selfdiagnostics SARS-CoV-2 IgM/IgG Antibody Assay Kit
Selfdiagnostics SARS- CoV-2 IgM/IgG Antibody Assay Kit is a lateral flow chromatographic immunoassay for the qualitative detection of IgM and IgG antibodies against SARS CoV-2 in human finger-stick or venipuncture whole blood, serum or plasma specimen in only 15 minutes.
Download IFU
Rapid Antibody Multitest

No special equipment,
transport or storage needed
200 clinical samples tested
3 times more than competitors.
Multiple samples
(finger-stick and venipuncture
whole blood, plasma, serum)

Ideal as a Triage and Screening Tool
- Doctors, nursing homes, clinics.
- Labs and COVID-19 wards in hospitals.
- Triages, emergency departments.
- Public screening sites etc.

In a clinical study with 200 clinical samples Selfdiagnostics SARS-CoV-2 IgM/IgG Antibody Assay Kit
was compared against confirmed cases of patients with SARS CoV-2 infection. We obtained the following results:
IgG sensitivity > 14 days from infection 97%
IgM sensitivity 7-14 days from infection 84%
Specificity 99%
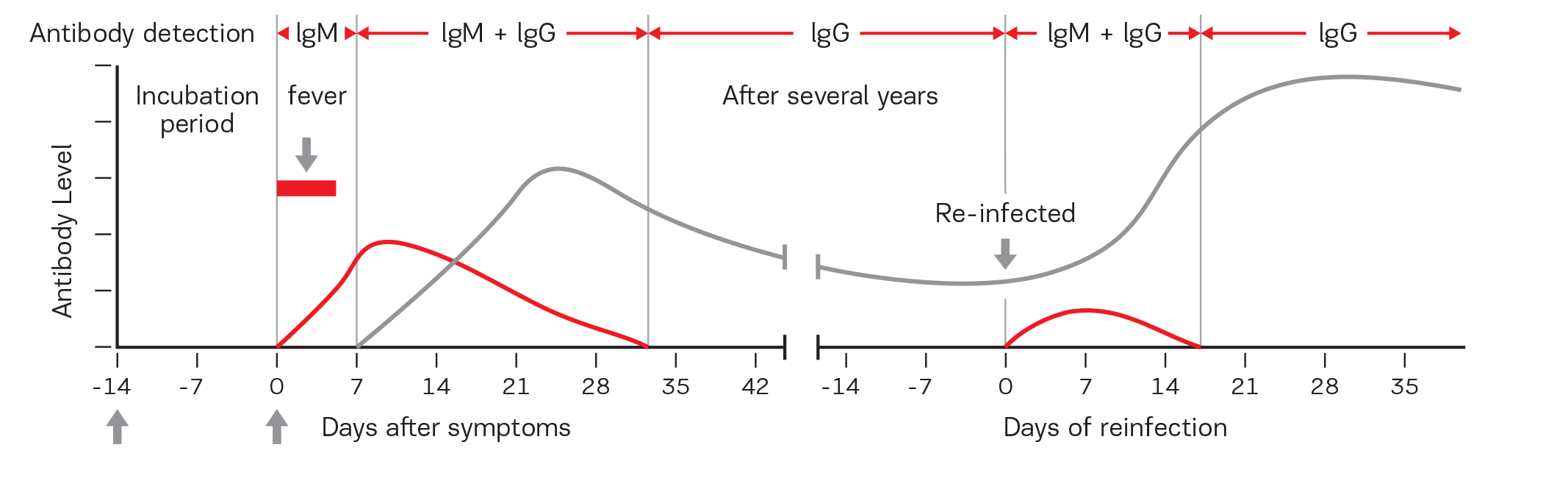
How does the Selfdiagnostics SARS-CoV-2 IgM/IgG Antibody Assay Kit work?
When the human body comes in contact with foreign antigens, IgM is the earliest antibody produced and its presence is detectable from day 3 after the onset of infection. Therefore, IgM can be used for reflecting whether the body is in an acute state of infection as a main indicator of early diagnosis.
Possible secondary SARS-CoV-2 infection is characterized by elevated SARS-CoV-2 IgG levels. In the majority of cases, IgM levels are also elevated. IgG levels may persist beyond the actual SARS-CoV2 infection. Therefore can indicate a past infection with SARS-CoV2.
Selfdiagnostics SARS-CoV-2 IgM/IgG Antibody Assay Kit
result interpretation
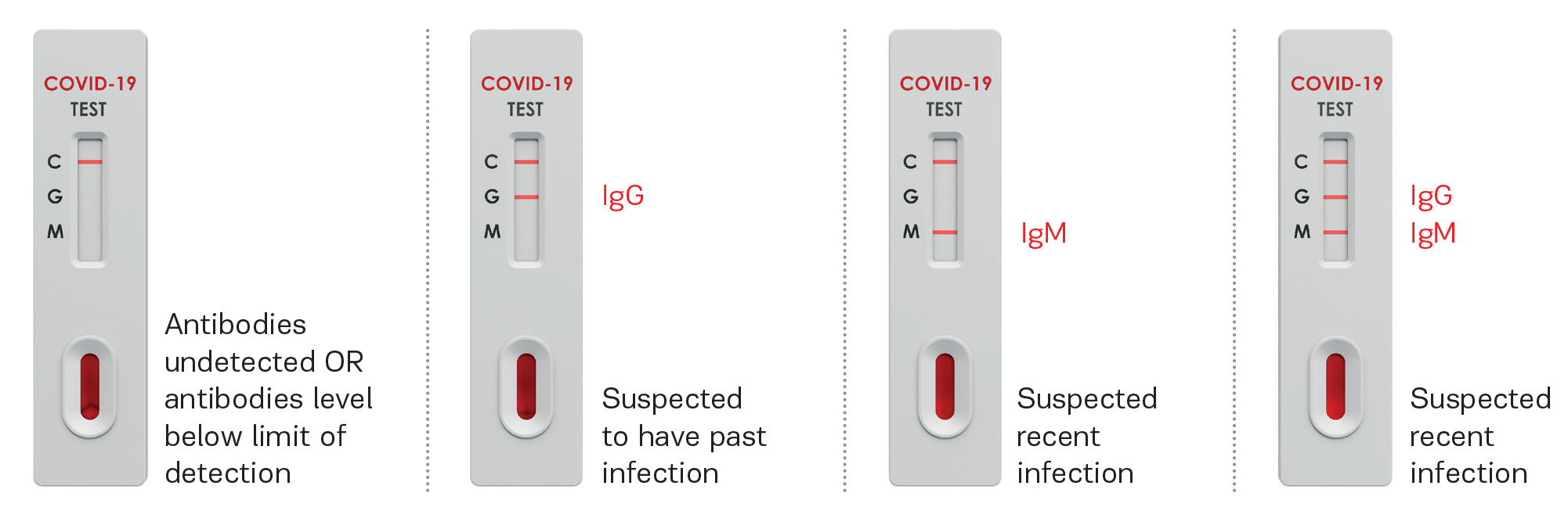
This test is not intended for to diagnose or exclude acute SARS-CoV-2 infection and direct testing should be performed for diagnosis.
Frequently Asked Questions
- What are IgG and IgM? –The presence of IgM is an indicator of early infection. IgG is a more specialised antibody that specifically binds to the SARS-CoV-2 virus. The presence of IgG is an indicator of later stage infection (usually 7 days or longer after infection).
- Is the test specific for COVID-19? -The IgG that the test detects is specific to COVID-19, so a positive result would indicate COVID-19 infection. The test can be used for primary and secondary diagnosis of COVID-19.
- What should I do if I test positive? -If you test positive, you should follow your country’s government’s advice on COVID-19 infection.
- What if I test negative? –A negative result means the biomarkers are not present in your blood, but you should still exercise caution, as you may still be in the early stages of infection (before IgM levels rise to a detectable level). If symptoms develop, follow the protocols as above and retest as necessary.
Why Selfdiagnostics?
Selfdiagnostics was founded in 2008 by entrepreneurs and scientists to develop new, easy to use, precise and low cost rapid Point-Of-Care tests to detect wide array of different diseases.
Selfdiagnostics offers the world’s smallest application of PCR technology to create tests that you can use in the comfort of your own home, without the need to see a doctor.
Selfdiagnostics is also medical equipment distributor, mainly in EU region.
Contact us
-
What is COVID-19?
Formerly, known as the ‘2019 novel coronavirus’ or ‘2019-nCoV.’ The COVID-19 virus is linked to the same family of viruses as Severe Acute Respiratory Syndrome (SARS) and some types of the common cold.
-
How dangerous is COVID-19?
For most of us, COVID-19 causes only a mild illness; for others, it can make them very ill and sometimes cause death. Senior people and those with pre-existing medical conditions (like high blood pressure, heart problems or diabetes) appear to be more vulnerable.
-
Symptoms of COVID-19
COVID-19 affects different people in various ways. Most of those only develop mild to moderate symptoms and recover without hospitalisation.
Most common symptoms are:
- Dry cough
- Fever
- Fatigue and tiredness
Less common symptoms are:
- Headaches
- Sore throats
- Diarrhoea
- Aches and pains
- Conjunctivitis
- Rashes on the skin
- Loss of taste or smell
-
Prevention of COVID-19
You can protect yourself and others by taking appropriate precautions advised by your local, state or governmental health authorities.
To prevent COVID-19 from spreading:
- Wash your hands frequently. Use soap and water, or an alcohol-based hand gel.
- Don’t touch your eyes, nose or mouth.
- Cover your nose and mouth using your bent elbow or a tissue if you need to cough or sneeze.
- Maintain a 2-metre distance from anyone who is coughing or sneezing.
- Wear a mask when physical distancing is not possible.
- Always stay home if you feel unwell.
- If you have a fever, cough or difficulty breathing, always seek medical attention.
Calling in advance allows your healthcare provider to direct you to the right health facility quickly. This protects you and prevents the spread of viruses and other infections.
Masks
Masks can help prevent the spread of the virus from the person wearing the mask to others. It should be noted that masks alone do not protect against COVID-19 and should be combined with physical distancing and excellent hand hygiene. Always follow the advice provided by your local health authority.
-
Treatments of COVID-19
To date, there are no medicines, treatments or vaccines for COVID-19. New treatments and vaccines are being tested through clinical trials.
Self-care
If you feel sick, you should rest, drink plenty of fluid, and eat nutritious food. Stay in a separate room from other family members, and use a dedicated bathroom if possible. Clean and disinfect frequently touched surfaces.
Everyone should keep a healthy lifestyle at home. Maintain a healthy diet, sleep, stay active, and make social contact with loved ones through the phone or internet. Children need extra love and attention from adults during difficult times. Keep to regular routines and schedules as much as possible.
It is normal to feel sad, stressed, or confused during a crisis. Talking to people you trust, such as friends and family, can help. If you feel overwhelmed, talk to a health worker or counsellor.
Medical treatments
If you have mild symptoms and are otherwise healthy, self-isolate and contact your medical provider or a COVID-19 information line for advice.
Seek medical care if you have a fever, a cough, and difficulty breathing. Call in advance.
-
Is there a test for COVID-19?
Many countries are simultaneously testing for SARS-CoV-2 antibodies at either population-level or testing specific groups, like health workers, close contacts of known cases, or within households. The WHO supports these studies, as they are critical for understanding the extent of – and risk factors associated with – infection.
Two kinds of COVID-19 tests are available: viral tests and antibody tests.
- A viral test detects if you have a present infection.
- An antibody test might identify if you had a past infection, but not necessarily a current detection. Having antibodies to the virus that causes COVID-19 may protect you from getting infected again with the virus.
-
How to get tested for COVID-19 infection
To determine if you have an infection, viral tests are used. Most people have mild illness and can recover at home without medical care. Contact your healthcare provider if your symptoms are getting worse or if you have questions about your health.
- Decisions about testing are made by state or local health departments or healthcare providers. You can visit their websites to look for the latest local information on testing.
- If you have symptoms of COVID-19 and want to get tested, call your healthcare provider first.
- If you have symptoms of COVID-19 and are not checked, it is important to stay home.
- If you have symptoms of COVID-19 and are not checked, it is important to stay home.
-
What should I do if I test positive for the coronavirus disease?
If you test positive, then you should isolate yourself. The people you have been in close contact with up to 2 days before you developed symptoms should be contacted and advised to seek a test too if they show signs of COVID-19.
The WHO also advises that all confirmed cases should be isolated in health facilities to prevent transmission and adequate care provided if countries can do this.
-
Buying and selling COVID-19 tests
Healthcare professionals always do COVID-19 tests. They are not designed to be used by people in their homes as they may give results that need to be interpreted by a trained and qualified healthcare professional.
Some tests are undertaken in a laboratory by specialist staff using specialist equipment. Other examinations require less equipment, are easier to use and can be performed by a fully trained and qualified healthcare professional.
A COVID-19 test that has a valid CE mark can be sold to members of the public and used by them privately.
Selfdiagnostics Deutschland SARS-CoV-2 IgM/IgG Antibody Assay Kit is one test that is permitted to be resold globally.